An atom is the smallest unit of ordinary matter that forms a chemical element. Atoms consist of:
1. Protons: Positively charged particles in the nucleus.
2. Neutrons: Particles with no charge in the nucleus.
3. Electrons: Negatively charged particles orbiting the nucleus.
 Key Characteristics
Key Characteristics
1. Atomic number: Number of protons defines the element.
2. Atomic mass: Total number of protons and neutrons.
Importance
1. Building blocks of matter: Atoms are the fundamental units of matter.
2. Chemical reactions: Atoms interact and form bonds with other atoms.
Atoms are the foundation of chemistry and understanding their structure and properties is crucial for understanding the world around us.
Structure of an Atom
An atom consists of three main parts: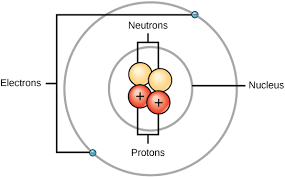 1. Protons
1. Protons
1. Positively charged particles
2. Located in the nucleus (center) of the atom
3. Determine the element’s identity (atomic number)
2. Neutrons
1. Particles with no charge (neutral)
2. Located in the nucleus along with protons
3. Contribute to the atom’s mass (atomic mass)
3. Electrons
1. Negatively charged particles
2. Orbit the nucleus in energy levels or shells
3. Participate in chemical bonding and reactions
Key Points
1. Nucleus: Protons and neutrons reside in the nucleus.
2. Electron cloud: Electrons occupy specific energy levels around the nucleus.
3. Atomic structure: Protons, neutrons, and electrons work together to form a stable atom.
Understanding the structure of atoms is essential for understanding chemistry and the behavior of matter.
- Definition of Atom
An atom is the smallest unit of ordinary matter that forms a chemical element, consisting of:
1. A nucleus containing protons and neutrons
2. Electrons orbiting the nucleus
Atoms are the building blocks of matter, and their unique properties determine the characteristics of elements.
Atomic Theory
The atomic theory states that matter is composed of small, indivisible particles called atoms. Key principles:
1. Atoms as building blocks: Atoms are the fundamental units of matter.
2. Indivisibility: Atoms cannot be created or destroyed, only rearranged.
3. Unique properties: Each element has unique atomic properties.
Development
1. Democritus: Proposed the concept of atoms in ancient Greece.
2. John Dalton: Developed the modern atomic theory in the 19th century.
Importance
1. Understanding matter: Atomic theory explains the properties and behavior of matter.
2. Chemical reactions: Atomic theory helps predict chemical reactions and bonding.
The atomic theory is a fundamental concept in chemistry and physics, shaping our understanding of the universe.
- History of Atomic Theory
The concept of atoms has evolved over time:
Ancient Era
1. Democritus (460-370 BCE): Proposed the idea of indivisible particles called atoms.
2. Epicurus (341-270 BCE): Further developed the concept of atoms.
Modern Era
1. John Dalton (1803): Developed the modern atomic theory, proposing that elements are composed of small, indivisible particles called atoms.
2. J.J. Thomson (1897): Discovered electrons, leading to the “plum pudding” model.
3. Ernest Rutherford (1911): Proposed the nuclear model, discovering the nucleus.
4. Niels Bohr (1913): Developed the Bohr model, introducing energy levels.
Key Milestones
1. Discovery of subatomic particles: Protons, neutrons, and electrons.
2. Atomic models: Evolution from plum pudding to nuclear and quantum models.
The history of atomic theory showcases human curiosity and scientific progress, transforming our understanding of matter.
- Atomic Number
The atomic number is the number of protons present in the nucleus of an atom, symbolized by Z. It determines the identity of an element and its chemical properties.

Definition
The atomic number is equal to the number of protons in an atom’s nucleus, which is also equal to the number of electrons in a neutral atom.
Importance
The atomic number defines the element’s position in the periodic table and determines its chemical behavior.
Examples
– Hydrogen: Z = 1 (1 proton)
– Carbon: Z = 6 (6 protons)
– Copper: Z = 29 (29 protons)
- Relationship with Mass Number
The mass number (A) is the sum of protons and neutrons in the nucleus. The atomic number determines the element’s identity, while the mass number determines the isotope.
The atomic number is a unique identifier for each element and plays a crucial role in understanding the properties and behavior of elements.
Atomic Structure
The atomic structure consists of:
1. Nucleus
1. Protons: Positively charged particles
2. Neutrons: Particles with no charge
2. Electron Cloud
1. Electrons: Negatively charged particles orbiting the nucleus
Key Components
1. Atomic Number (Z): Number of protons
2. Mass Number (A): Total number of protons and neutrons
Importance
1. Chemical Properties: Determined by atomic structure
2. Chemical Bonding: Atoms interact through electrons
Understanding atomic structure is essential for understanding chemistry and the behavior of matter.
More read..,
- Ecosystem and Biodiversity?
- Electromagnetic Spectrum?
- Difference between mitosis and meiosis?
- Biogas?
- NDA Application form ?